Expansion Driven Heating or Cooling of Gases
Such an occurrence is commonly referred to as the Joule-Thomson (J-T) effect or expansion named after the 19th century physicists James Prescott Joule and William Thomson (a.k.a. Lord Kelvin) who first collaboratively described this physical phenomenon.
Expansion Driven Heating or Cooling of Gases

Conversely, J-T expansion of an ideal gas is not possible since ideal gas enthalpy only depends on temperature, and, hence, expansion of an ideal gas under the same conditions is always isothermal.
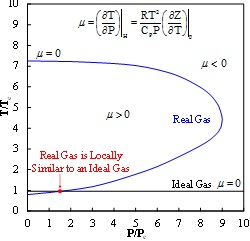
Consequently, the temperature and pressure of a real gas undergoing J-T expansion is related through a P-T envelope or inversion curve dependent on the J-T coefficient, m, while for an ideal gas mis always zero.
Isothermal expansion occurs when m= 0 and the process lies on the J-T inversion curve. Accordingly, closed loop process schemes can be designed to utilize such expansion driven auto-refrigeration or self-heating behavior of gases and even highly volatile liquids to manipulate the thermal state or quality of a fluid stream and facilitate a process change.
In such a scheme, liquid is drawn from the bottom of the tank using a pump, passed through a process cooler to remove heat added from bulk fluid conveyance and night-to-day heating of the tank contents, and then back into the tank through a control valve followed by a distributor lance or spray nozzle with it flashing by J-T expansion to a vapor having a temperature substantially lower than the saturation temperature of the mixture in the tank which cools the vapor space and reduces the tank pressure through vapor condensation.
Furthermore, the generation of super-heated steam can be achieved via J-T expansion when saturated or wet steam is flashed to a lower pressure through a control valve where a lower pressure and temperature steam will be produced with the temperature above the saturation value.[3] Alternatively, high ratio expansion of purified hydrogen gas during filling of industrial hydrogen storage tanks causes the H2 to heat which can be utilized for other process heating by cross-stream heat exchange.[4]
